www.apjph.comwww.apjph.com

Ref Number = ASPR0002
The Role of Gut Health in Early Life to Prevent Stunting
Reza Ranuh Department of Child Health - Dr. Soetomo Hospital Medical Faculty Universitas Airlangga - Surabaya Email : rezagunadi@gmail.com
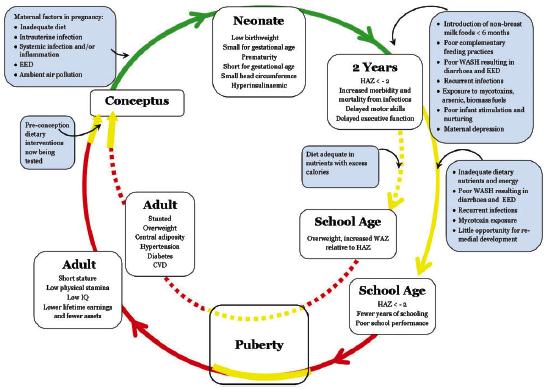
There is a growing consensus that pregnancy and the first 2 years of life are the most critical time period for interventions to improve child growth and development. This is the age of greatest vulnerability to undernutrition and infection. Potentially irreversible long-term physical and mental damage due to undernutrition occurs during this period (1,2,3,4). Linear growth failure is the most common form of undernutrition globally. Stunting in which multiple pathological changes marked by linear growth retardation in early life are associated with increased morbidity and mortality, reduced physical, neurodevelopmental and economic capacity and an elevated risk of metabolic disease into adulthood (5,6). With an estimated 165 million children below 5 years of age affected, stunting has been identified as a major public health priority, and there are ambitious targets to reduce the prevalence of stunting by 40% between 2010 and 2025 (1,2). The pathogenesis of stunting is poorly understood. Prenatal and postnatal nutritional deficits, enteric and systemic infections clearly contribute, but recent findings implicate a central role for environmental enteric dysfunction (EED), a generalized disturbance of small intestinal structure and function found at a high prevalence in children living under unsanitary conditions. Mechanisms contributing to growth failure in EED include intestinal leakiness and heightened permeability, gut inflammation, dysbiosis and bacterial translocation, systemic inflammation, and nutrient malabsorption (Fig.1)(1,2,10) ASPR0002 - 1 Figure 1. The multifactorial causes of stunting syndrome in early life Prendergast and Humphrey. Paediatrics and International Child Health 2014 VOL. 34 NO. 4 The assembly and succession of the early-life microbiota plays important roles in growth and maturation of the endocrine, mucosal immune, central nervous systems and gut health developmental pathways during early life to prevent stunting (1,2). The microbiota of the healthy newborn closely matches the maternal stool, vaginal, or skin microbiota, depending on delivery mode. The first colonizers of the infant gut microbiota are typically facultative anaerobes, followed by the accumulation of obligate anaerobes, including Bifidobacterium, Bacteroides, and Clostridium during the following 6 months. The diversity of the microbiota remains narrow in early infancy and is dominated by species involved in human milk oligosaccharide (HMO) metabolism in breastfed infants. It has been estimated that 25?30% of the infant bacterial microbiota originates from breast milk. Germ-free mice exhibit significantly reduced weight and length prior to weaning compared with conventionally raised animals. This may be due to a number of factors, including reduced capacity for energy harvest from the diet; however, it has also been theorized that microbiota-induced interactions with insulin-like growth factor 1 (IGF-1), that remain uncharacterized, may also play a role in early-life growth (7,8). The programmed maturation of the microbiota in early childhood appears to influence linear as well as ponderal growth. A small study in India examined the longitudinal succession of the infant microbiota from birth to 2 years, reporting that reduced relative abundance of B. longum and Lactobacillus mucosae in addition to elevated relative abundance of Desulfovibrio spp. was associated with stunting. The commensal gut microbiota regulates a number of processes that affect child growth in the first 1000 days. (Fig.2) ASPR0002 - 2 Figure 2. The Pathways by Which Microbes in the Intestinal Lumen Interact with Host Growth in Healthy versus Malnourished Children. Trends in Microbiology, February 2019, Vol. 27, No. 2 (6). The structural and functional integrity of the intestinal barrier (mucus layer, antimicrobial peptides, epithelium and tight junctions) is tightly regulated by gut microbial composition in healthy infants but becomes perturbed in undernutrition (1, 2, 6, 7, 8). A healthy microbiome and the innate immune system maintains immune homeostasis also provides colonization resistance against invading pathogens (6, 7,8). The host microbiome also regulates somatotropic axis (GH/IGF-1) activity to stimulate growth in early life, through mechanisms that remain unknown. The microbiome plays a critical role in nutrient and host metabolism, thereby affecting digestion, absorption, and energy storage. A dysbiotic microbiome in early life may impair each of these pathways related to growth whereby an immature microbiota fails to protect the intestinal barrier leading to villous blunting, mucus degradation, intestinal permeability, and impaired immune responses. These intestinal impairments may contribute to environmental enteric dysfunction (EED), chronic systemic inflammation, infectious morbidity and diarrhea, each of which may impair the trajectories of growth. A combination of ?hits? may be required to induce EED and undernutrition phenotypes, including both an insufficient or inadequate diet (1), pathogen carriage (2), and/or a dysbiotic microbiome (3). Microbiome dysbiosis also may impair metabolism of key nutrients, including essential amino acids, thereby preventing normal growth. A disturbed gut microbiota composition may impair the normal production of growth hormones (6, 9,10).
Keywords: Gut health; Gut microbiota; Stunting

Refference:
1. Andrew J. Prendergast & Jean H. Humphrey.,(2014) The stunting syndrome in developing countries. Paediatrics and International Child Health 2014 Vol. 342. Andrew J. Prendergast, Jean H. Humphrey. Stunting Persists despite Optimal Feeding: Are Toilets Part of the Solution?.,(2015) Nestlé Nutr Inst Workshop Ser, Nestec Ltd., Vevey/S. Karger AG., Basel. vol 81, pp 99–110, ( DOI: 10.1159/000365807 )3. JeffreyI.Gordon, Kathryn G. Dewey, David A. Mills, Ruslan M.Medzhitov.,(2012) The Human Gut Microbiota and Undernutrition. www.ScienceTranslationalMedicine.org Vol 4 Issue 137 137ps124. Reza Ranuh, Alpha Fardah Athiyyah, Andy Darma, Vitria Prasetyo Risky, Wibi Riawan, Ingrid S. Surono, Subijanto Marto Sudarmo,. (2019) Effect of the probiotic Lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: role of intestinal microbiota on the gut-brain axis. Iran. J. Microbiol. Volume 11 Number 2 (April 2019) 145-1505. Tariq Jamal Khan, Mohammed Nihal Hasan, Esam I. Azhar, Muhammad Yasir., (2019) Association of gut dysbiosis with intestinal metabolites in response to antibiotic treatment. Human Microbiome Journal 11 1000546. Ruairi C. Robertson, Amee R. Manges, B. Brett Finlay and Andrew J. Prendergast., (2019) The Human Microbiome and Child Growth – First 1000 Days and Beyond. Trends in Microbiology, February Vol. 27, No. 27. Duy M. Dinh, Balamurugan Ramadass, Deepthi Kattula, Rajiv Sarkar, Philip Braunstein, Albert Tai, Christine A. Wanke, Soha Hassoun, Anne V. Kane, Elena N. Naumova, Gagandeep Kang, Honorine D. Ward.,(2016) Longitudinal Analysis of the Intestinal Microbiota in Persistently Stunted Young Children in South India. PLOS ONE DOI:10.1371/journal.pone.0155405 May 268. Mduduzi N.N. Mbuya and Jean H. Humphrey., (2016) Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: an opportunity for stunting reduction in developing countries. Maternal & Child Nutrition published by John Wiley & Sons Ltd., 12 (Suppl. 1), pp. 106–120 DOI: 10.1111/mcn.122209. Richard L. Guerrant, Mark D. DeBoer, Sean R. Moore, Rebecca J. Scharf and Aldo A. M. Lima.,(2012) The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. nature reviews | Gastroenterology & Hepatology. Advance online publication, December10. Victor Owino, PhD, Tahmeed Ahmed, Michael Freemark, Paul Kelly, Alexander Loy, Mark Manary, Cornelia Loechl., (2016) Environmental Enteric Dysfunction and Growth Failure/Stunting in Global Child Health. PEDIATRICS, December Volume 1 38, number 6
Disclaimer: The Views and opinions expressed in the articles are of the authors and not of the journal.
Editor-In-Chief
Journal Office
Mid City Hospital, 3-A Shadman II
Jail Road, Lahore ,Pakistan
Mid City Hospital, 3-A Shadman II
Jail Road, Lahore ,Pakistan
Managing Editor
Dr. Intan Juliana Abd Hamid
Support & Help
Support & Help
Assistant Editor
Dr. Sadia Shabbir Hussain
Support & Help
Support & Help
Digital Content Editor
Dr. Khalid Masud
Administrator
Administrator